Researchers from the Laboratory for Hydraulic Machines (LVTS) and the Department of Polymer Chemistry and Technology at the National Institute of Chemistry (KI) have degraded hydroxypropyl methylcellulose using acoustic and hydrodynamic cavitation. The results of the study were published in the journal Ultrasonics Sonochemistry (IF=8.7).
When cavitation bubble collapses, the energy is released in an extremely confined area in space and time, resulting in the mechanical and chemical phenomena associated with cavitation. These phenomena can cause the cleavage of glycosidic bonds. The researchers compared the efficiency of polysaccharide degradation with two types of cavitation: a) acoustic cavitation, where cavitation is caused by acoustic sound waves, and b) hydrodynamic cavitation, where cavitation is caused by local increase in fluid velocity.
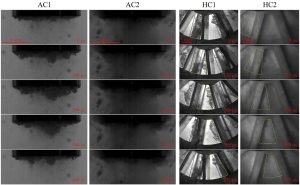
Figure 1: Appearance of cavitation: AC1 – acoustic cavitation without added polymer, AC2 – acoustic cavitation with added polymer, HC1 – hydrodynamic cavitation without added polymer, HC2 – hydrodynamic cavitation with added polymer.
Polysaccharide treatment methods that lead to lower molar masses and lower dispersity require long treatment times (e.g. 24 hours). In the study, researchers used cavitation and an addition of oxidant to reduce the average molar mass of the polysaccharide by 98.8% in 30 minutes while also reducing dispersity.
The study’s results confirm that cavitation treatment could be used as an alternative to the commonly used chemical and enzymatic methods to cleave the glycosidic bonds. The knowledge gained in this study can serve as a basis for the degradation of other natural polysaccharides such as hyaluronic acid and chitosan.
