Freshwater scarcity has become a global challenge that threatens sustainable development and human safety. The atmosphere contains around six times as much water as all the world’s river systems combined. The atmospheric water harvesting technology allows large quantities of fresh water to be extracted anywhere and at any time, thereby providing a sustainable solution to address water scarcity.
Assist. Prof. Dr. Primož Poredoš from the Faculty of Mechanical Engineering in Ljubljana and researchers from the ITEWA innovation team from the Shanghai Jiao Tong University and the Department of Materials Science and Engineering of the National University of Singapore co-authored a review scientific article that analyzes the interaction between hygroscopic salts, water and saline solutions in hygroscopic salt-doped composite adsorbents from the perspective of thermodynamics. The article establishes a relationship between key structural parameters – such as type and content of hygroscopic salts, pore structure and carrying capacity of the matrix – and the final water uptake capacity. The review also proposes the theoretical design framework to guide adsorption capacity, enthalpy of desorption, kinetics and stability of adsorbents, providing a uniform method for verification and regulation in the development of adsorption materials for atmospheric water harvesting and related areas.
Adsorption capacity of hygroscopic materials (i. e. sorbents) determines their ability to extract water vapour from the environment and is a key factor of the efficiency of sorption capture of atmospheric water. However, for most of the salt-doped composite sorbents described so far, the qualitative selection of synthesis parameters and unclear interactions between the components greatly hinder the fine-tuning and prediction of their adsorption behaviour. In addition, the integration of these technologies with new energy approaches, such as daytime radiative cooling and 3D printing (Figure 1), calls for even more fine-tuned design methods to fully exploit their development potential.
This ground-breaking research was published in Nature Reviews Materials (IF = 86.2), one of the most distinguished scientific journals in the field of materials, issued by Springer Nature.

Picture 1. Evaluation of sorption capacity in relation to salt content, including typical capillary HSCMs with their fabrication methods, optical and microscopic images, and techniques for incorporating hygroscopic salts into gel matrices to synthesize composite hygroscopic sorption materials.
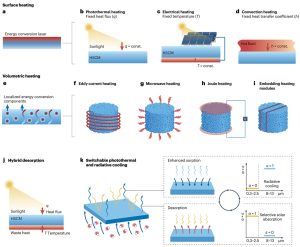
Picture 2. Methods of heat and cooling energy supply for enhancing sorption and desorption processes.
To design material’s water-binding capacities (adsorption capacities), the researchers theoretically calculated how much moisture a material can adsorb. This property is called water uptake (gram of water per gram of dry sorption material). This was done using a multi-stage reaction theory that considers three key stages:
- Adsorption – hydration (water binding into salt’s crystal lattice),
- Deliquescence (water uptake when salt from air adsorbs so much water to form a saline solution),
- Absorption (absorption of water into a saline solution).
The calculations also took into account the equilibrium vapour pressure, which means that the researchers determined how atmospheric water vapour behaves until it reaches balance with the material. This allowed them to determine very accurately how much water the material can accept in different conditions.
To design the desorption enthalpy (the energy required to break the bonds holding adsorbed water molecules to a material), they calculated the changes of energy in different stages of the drying process. They particularly distinguished between:
- enthalpy of solution concentration (the energy released or used when salt solution is concentrated), and
- evaporation enthalpy (the energy needed to convert water from a liquid to a gaseous state).
In this way, they can accurately determine what environmental conditions (e.g. temperature or humidity) a material needs for effective drying and re-adsorption.
In designing the rate of adsorption (adsorption kinetics), the researchers unified the explanation of how fast water penetrates gel and non-gel matrices. They examined which structural factors (e.g. pore size, particle shape and distribution) affect the rate of moisture adsorption and how this is reflected in the overall performance of the material. This resulted in a theoretical basis for evaluating and comparing different materials.
For the stability of the material, the researchers analyzed volume expansion of hygroscopic salts after water adsorption and the effect on the strength or carrying capacity of the matrix. This is important because the structure must remain mechanically stable despite the recurrent adsorption (moisture adsorption) and desorption (drying) cycles.
From an energy point of view, the reserachers examined how the input of cooling during adsorption and heat input during desorption can increase the efficiency of the entire process (adsorption and drying cycle). They also proposed concrete methods for improving both steps and strategies that could further support both processes.
One of the key advances is the use of daytime radiative cooling – i.e. passive cooling of terrestrial bodies with simultaneous reflection of incident solar radiation (over 95% solar reflectivity) and heat dissipation into space through the atmospheric window (thermal emissivity in the atmospheric window of at least 85%) without energy consumption. If the outer layer of the material is wisely designed so that it can switch, if needed, between radiative cooling and solar heat absorption, the material not only absorbs moisture more efficiently, but can also release it sustainably. Such a process allows for a comprehensive use of sustainable and renewable energy of the sun and the cold space.
