Researchers from the Laboratory for Hydraulic Machines (LVTS), in collaboration with the Department of Polymer Chemistry and Technology, the Department of Inorganic Chemistry and Technology, and the Department of Catalysis and Chemical Reaction Engineering at the Institute of Chemistry (KI), as well as the Faculty of Pharmacy (FFA), prepared low molecular weight chitosan using cavitation bubbles. The results of the study were published in the journal Ultrasonics Sonochemistry (IF=9.7).
When cavitation bubble collapses, energy is released in an extremely confined area in space and time, resulting in the mechanical and chemical phenomena associated with cavitation. These phenomena can cause the cleavage of glycosidic bonds. In this study, researchers investigated the influence of cavitation intensity, H2O2 concentration, sample preparation, and medium pH on the efficiency of chitosan degradation.
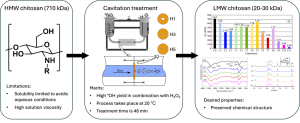
Figure 1: Graphical abstract of the study
Conventional methods for preparing low molecular weight chitosan require high temperatures or are time-consuming and often damage structural units. In this study, using cavitation and the addition of an oxidant, the researchers reduced the weight-average molecular weight of chitosan by 97.2% in 48 minutes without altering its backbone structure, ensuring preservation of functional properties.
The results of the study reaffirm that cavitation treatment could be used to cleave glycosidic bonds in polysaccharides. This method offers a faster, milder, and more sustainable alternative to chemical and enzymatic approaches, highlighting its potential for scalable and eco-friendly production of bioactive low molecular weight chitosan.
